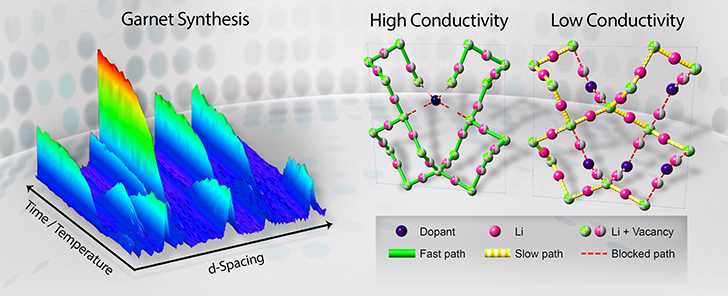
Neutron diffraction determines the lithium vacancy distribution in the garnet lattice and reveals the governing mechanism of highly conductive pathways
Announced December 21, 2015, a new study conducted at Oak Ridge National Laboratory's Spallation Neutron Source (SNS), a Department of Energy Office of Science user facility, has revealed promising results that could dramatically boost the performance of solid-state electrolytes, potentially leading to a safer, more efficient battery.
Solid electrolyte-based lithium-ion batteries consist of two electrodes, a positive and a negative, and an electrolyte in the middle, forming the battery's core, which facilitates the movement of ions traveling back and forth between the electrodes. In order to achieve a desired level of conductivity in the electrolyte, ions require vacancies in the crystal structure, or tunnels for the ions to hop to and from, a connecting of dots.
Lithium lanthanum zirconates or materials with a garnet structure are favorable for application as electrolytes because they promote fast lithium transport. However, synthesized garnets often develop unwanted low-conductivity secondary phases, which in some cases can be detrimental to electrolytic performance.
The scientists monitored the low-conductivity phases' formation during the thermal process and found that it could be mitigated by doping the material, adding trace amounts of various elements that have high valences, or an affinity to create bonds, to reduce the effect. Being able to both suppress the formation of those unwanted phases and increase the number of useful vacancies for ion transport proved to be the key to unlocking garnets with high electrolytic performance.